Chlorates & Chlorites

Chlorates & Chlorites
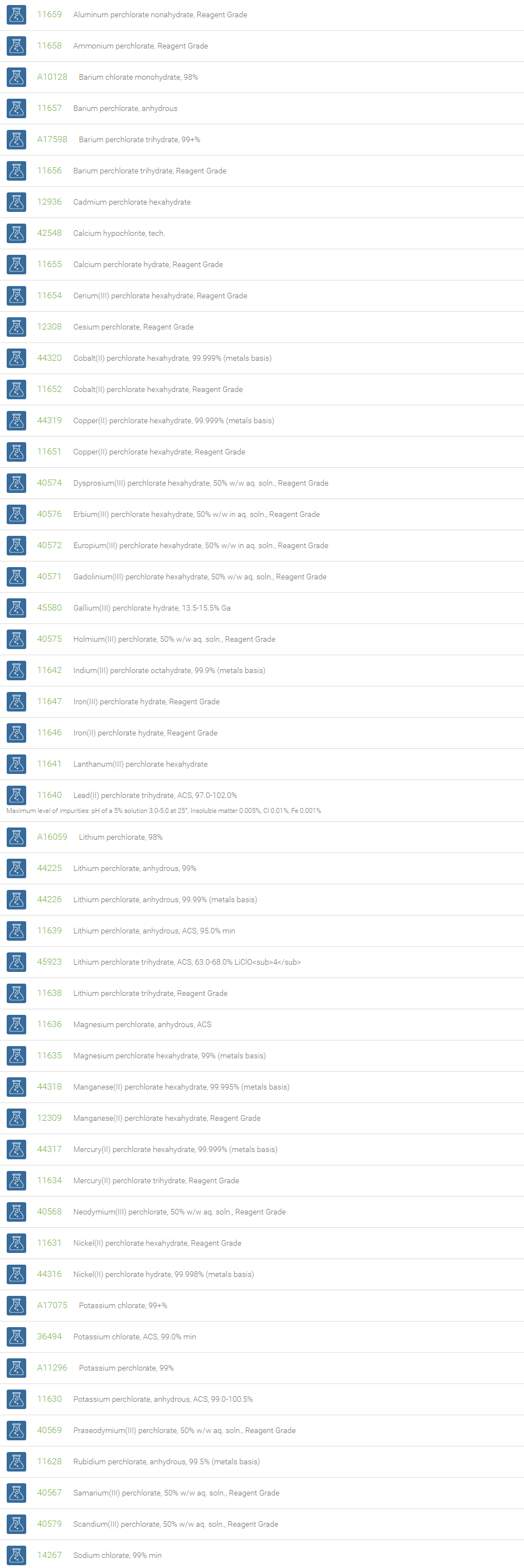
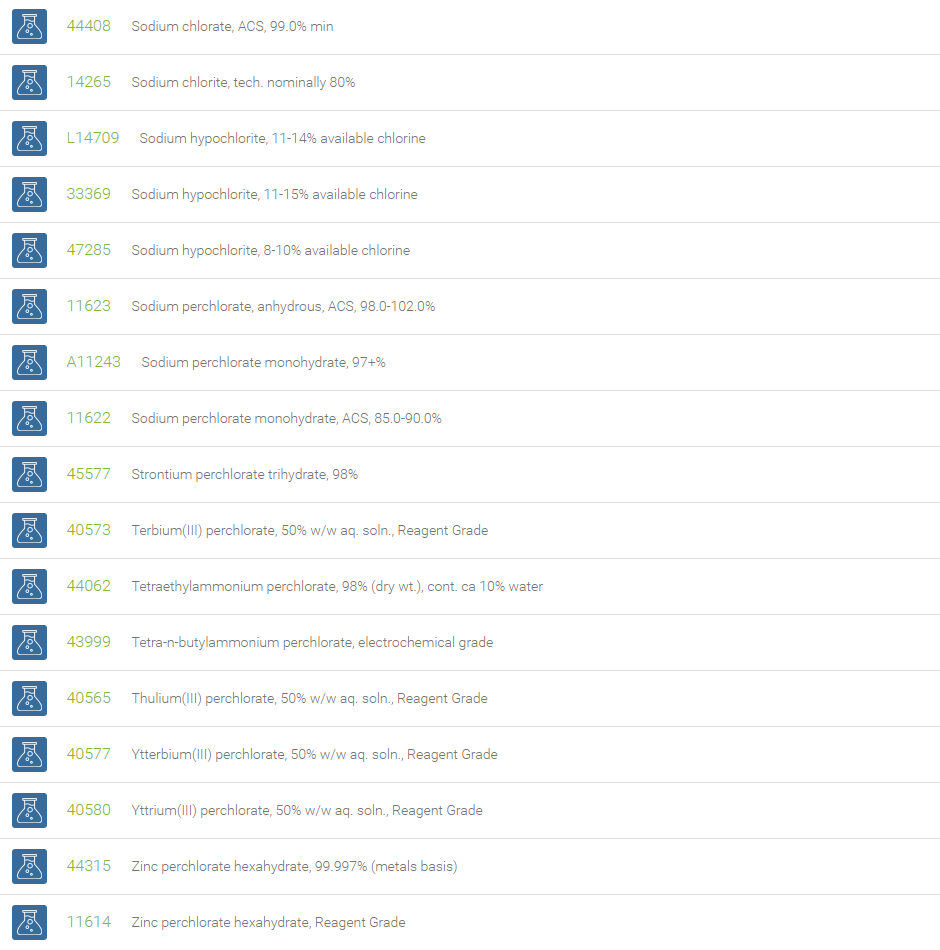
Chlorates are chemical compounds containing the anion having the formula ClO3–. In these compounds, chlorine atom is in the +5 oxidation state. Chlorates are the salts of chloric acid and are very strong oxidizers. Chlorates can be used as a source of oxygen. Mixtures of chlorate salts with virtually any combustible material (sugar, sawdust, metals, etc.) will readily deflagrate. Most pyrotechnic applications which formerly used chlorates in the past now use the more stable perchlorates instead. Sodium chlorate is used to manufacture chlorine oxide. Chlorates are used in the manufacture of dyes, matches, fireworks, disinfectants, and for tanning and finishing leather.
Chlorite is a compound that contains the chlorine dioxide anion (ClO2–), with chlorine in the +3 oxidation state. Chlorites are salts of chlorous acid. Sodium chlorite, NaClO2, is stable, inexpensive and commercially available. Sodium chlorite is used for on-site production of chlorine dioxide. It has also been used as a bleaching agent in the production of paper, textiles and straw products, in the manufacture of waxes, shellacs and varnishes, in disinfectant formulations, in sterilization, and in etching printed circuit boards. It is also used for disinfection in water treatment plants. Sodium chlorite also finds application as a component of contact lens cleaning solution.