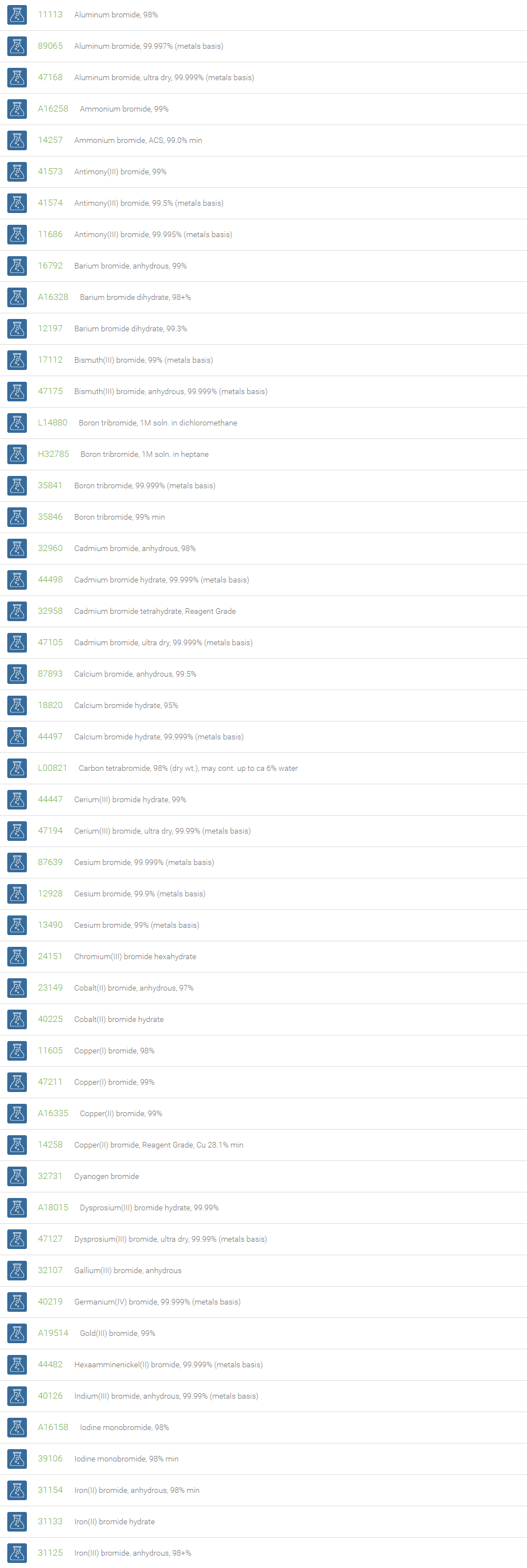
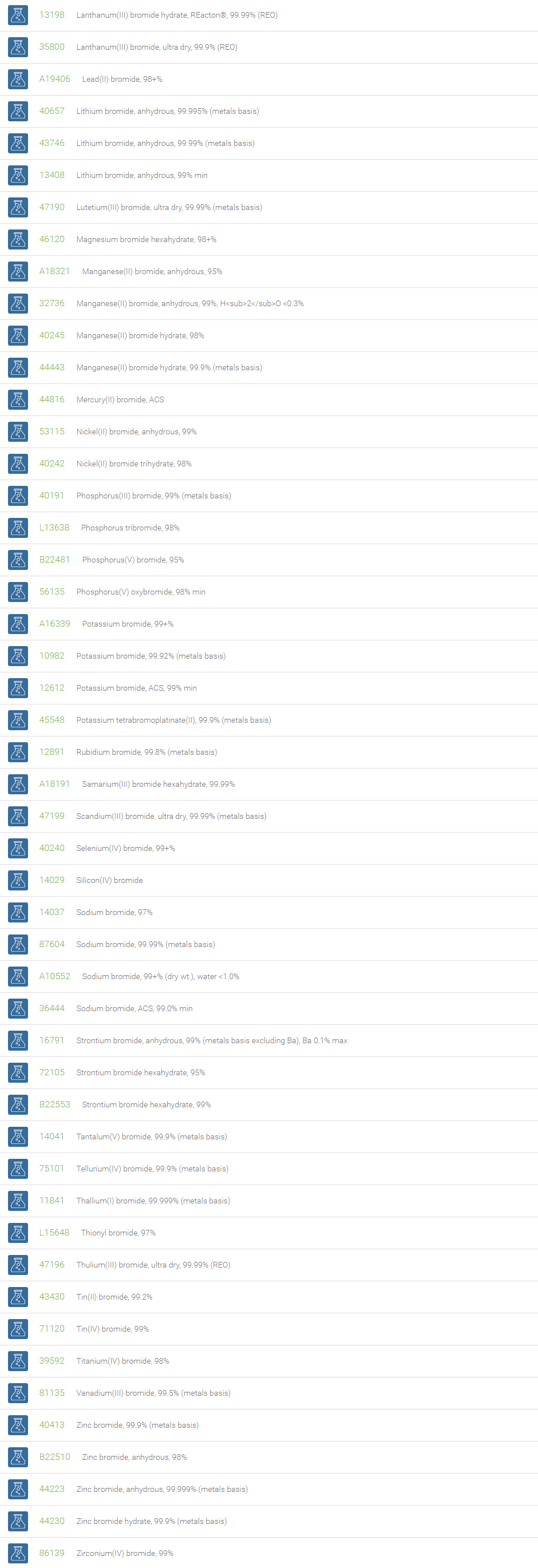
Inorganic Bromides

Inorganic Bromides
Inorganic bromides are group of compounds that contain a bromide ion or ligand with an ionic charge of ?1, and a more electropositive element or radical. Bromide is commonly found in nature along with sodium chloride. Most metal bromides are water soluble; exceptions are bromides of copper, lead, mercury, and silver, which are very slightly soluble in water.
Potassium bromide and sodium bromide are the familiar bromides used in medicine as sedatives. Magnesium bromide, found in seawater, is a source of pure bromine. Silver bromide is one of the light-sensitive silver salts used in films, plates, and printing papers for photography. The bromide ion is antiepileptic, and bromide salts are still used as such, particularly in veterinary medicine. Bromide is used as treatment for bipolar disorder. Bromide is needed by eosinophils, which use it to generate antiparasitic brominating compounds such as hypobromite. The bromide ion is a water disinfectant byproduct when ozone, or possibly hypochlorite (bleach), is used as a disinfectant. The bromide ion itself is sometimes used as a water disinfectant because in water it forms hypobromous acid (HOBr), which is a strong disinfectant.