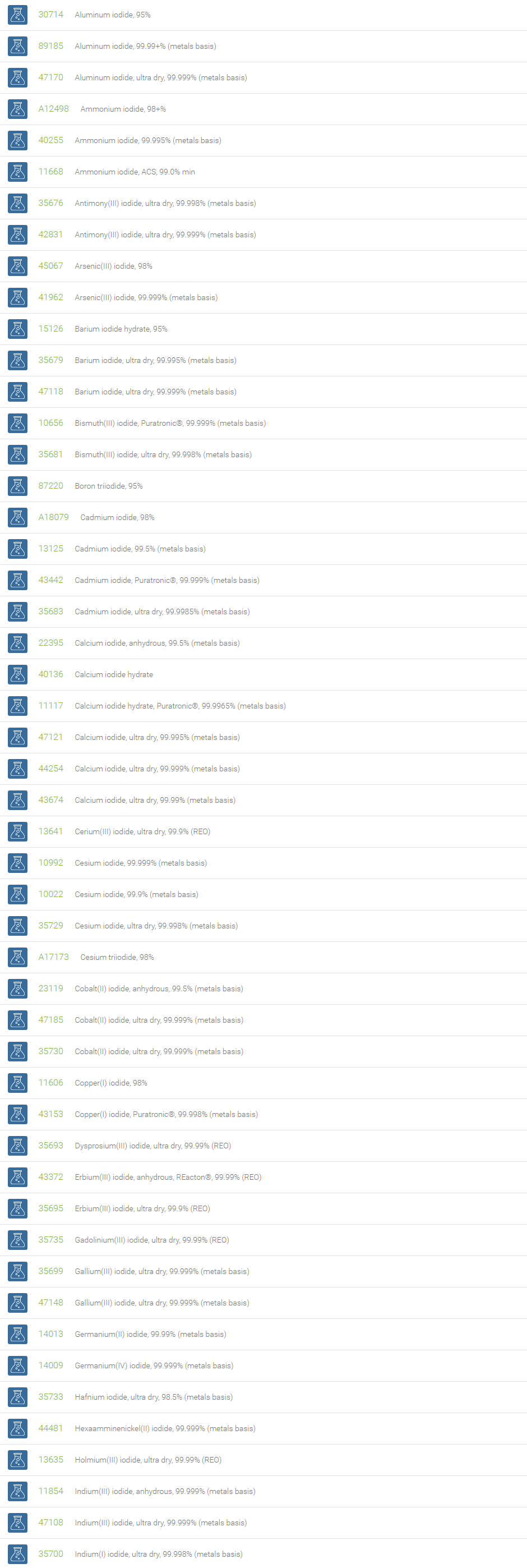
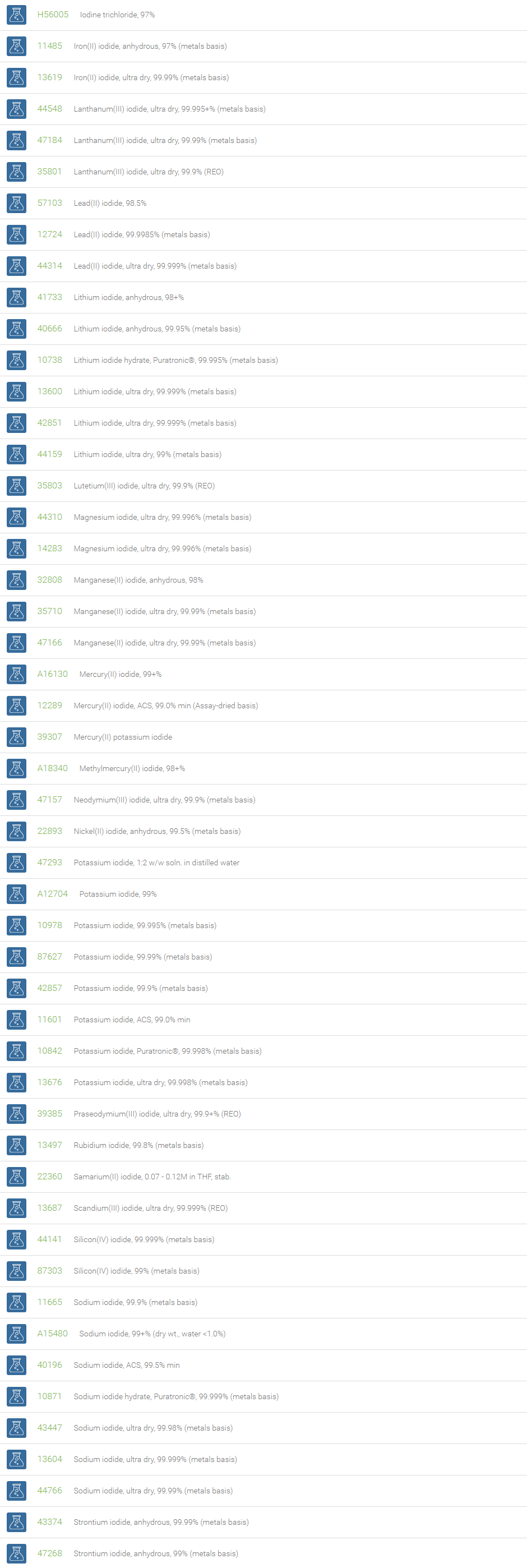
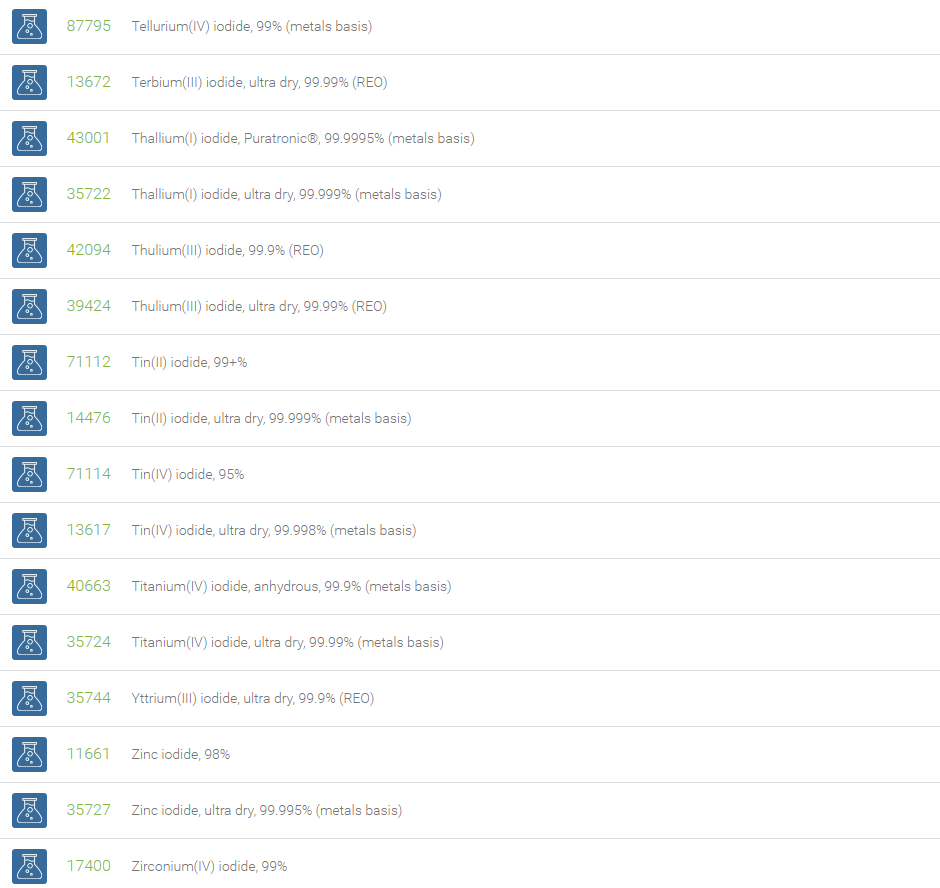
Inorganic Iodides

Inorganic Iodides
Compounds with iodine in formal oxidation state ?1 are called iodides. The iodide ion, I?, is one of the largest monoatomic anions. Examples include ionic compounds such as cesium iodide, potassium iodide, magnesium iodide, zinc iodide and silver iodide. Iodide can function as a nucleophile and an antioxidant reducing species that can destroy reactive oxygen species such as hydrogen peroxide.
Iodide salts are mild reducing agents and many react with oxygen to give iodine. Iodide compounds are used in photography, lithography, electroplating, manufacture of phosphors, Grignard reactions, and cloud seeding. Magnesium iodide in the Baylis-Hillman reaction gives (Z)-vinyl compounds (Tietze, Lutz-Friedjan, et al., Domino Reactions in Organic Synthesis, Wiley-VCH, 2006, 59). Copper iodide is useful in the exchange of bromides attached to sp2 carbon atom in organic compounds with iodides. NaI is useful in the aromatic Finkelstein reaction. Among halides, aryl iodides are preferred to form carboncarbon and carbonheteroatom bonds in process such as the Heck, Stille, Sonogashira and Suzuki coupling reactions. Cobalt(II) iodide is used as a catalyst in carbonylations. It catalyzes the reaction of diketene with Grignard reagents, useful for the synthesis of terpenoids. Strontium iodide finds use as a scintillation gamma radiation detector. Cesium iodide is often used as the input phosphor of an x-ray image intensifier tube, & as beam splitter in Fourier Transform Infrared (FT-IR) spectrometers. CuI is used in the detection of mercury vapors through visual color change into brown. Zinc iodide is often used as an x-ray opaque penetrant in industrial radiography