Inorganic Phosphates

Inorganic Phosphates
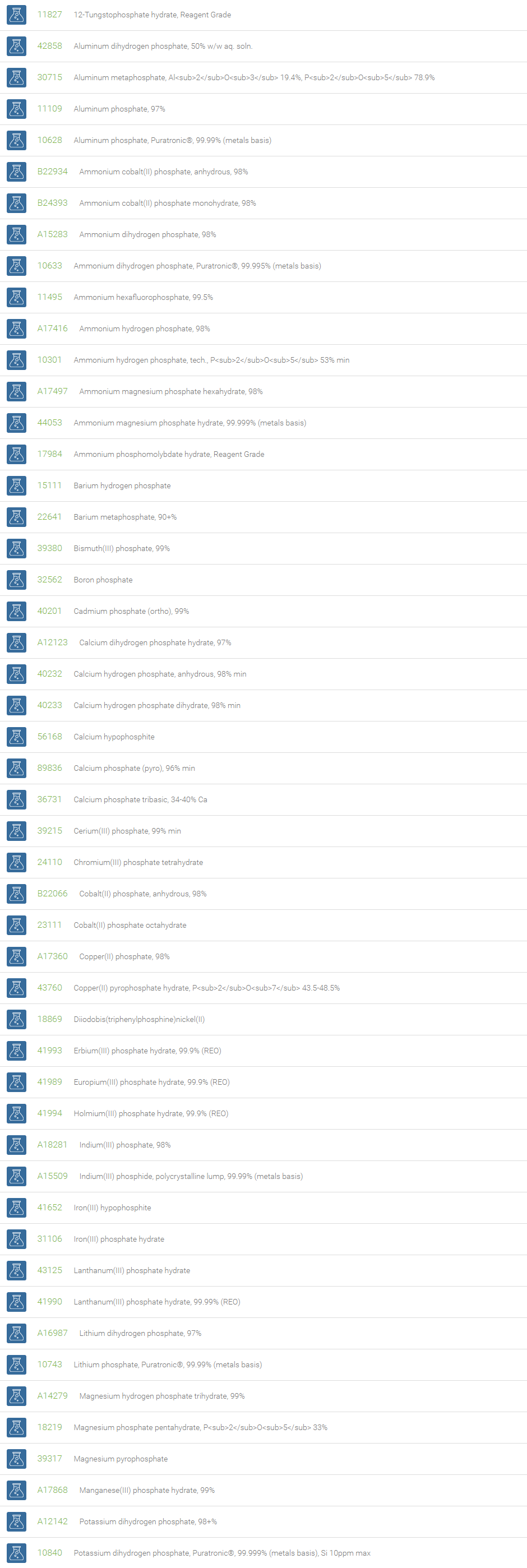
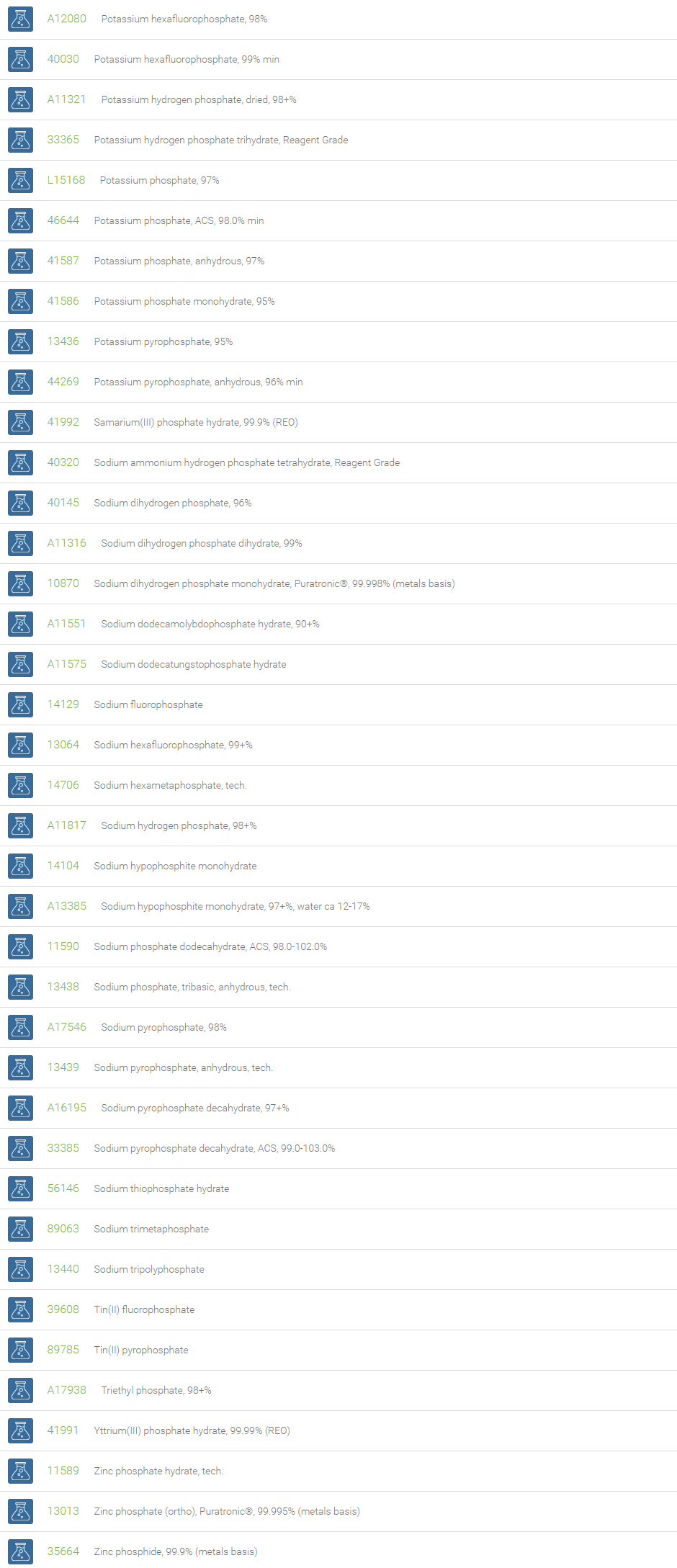
An inorganic phosphate (PO43-) is a salt of phosphoric acid with metal ions. It consists of one central phosphorus atom surrounded by four oxygen atoms in a tetrahedral arrangement. Inorganic phosphates occur naturally in many forms and are usually combined with other elements (e.g., metals such as sodium, potassium, calcium and aluminum). Inorganic phosphates are present in all living organisms and are required to support life. Inorganic phosphate in aqueous solution exists primarily as H2PO4– or HPO42-, and is an effective buffer.
Inorganic phosphates can be created by the hydrolysis of pyrophosphate. At elevated temperatures in the solid state, phosphates can condense to form pyrophosphates. Phosphate conversion coatings are applied on several metals for preventing corrosion and lubricity. Phosphates of manganese, zinc, and iron are employed in such conversion coatings. In analytical chemistry, phosphates of sodium and potassium are extensively used as a buffer for mobile phases. Diammonium phosphate (DAP) and monoammonium phosphate (MAP) are the two major NP fertilizers. Monobasic and dibasic phosphates are used in milk to prevent coagulation during processing, in powdered products as an anti-caking additive, in desserts and puddings, and in detergents. These phosphates are useful for the preventing calcium scaling. Monosodium phosphate and disodium phosphate are used as a saline laxative to treat constipation.