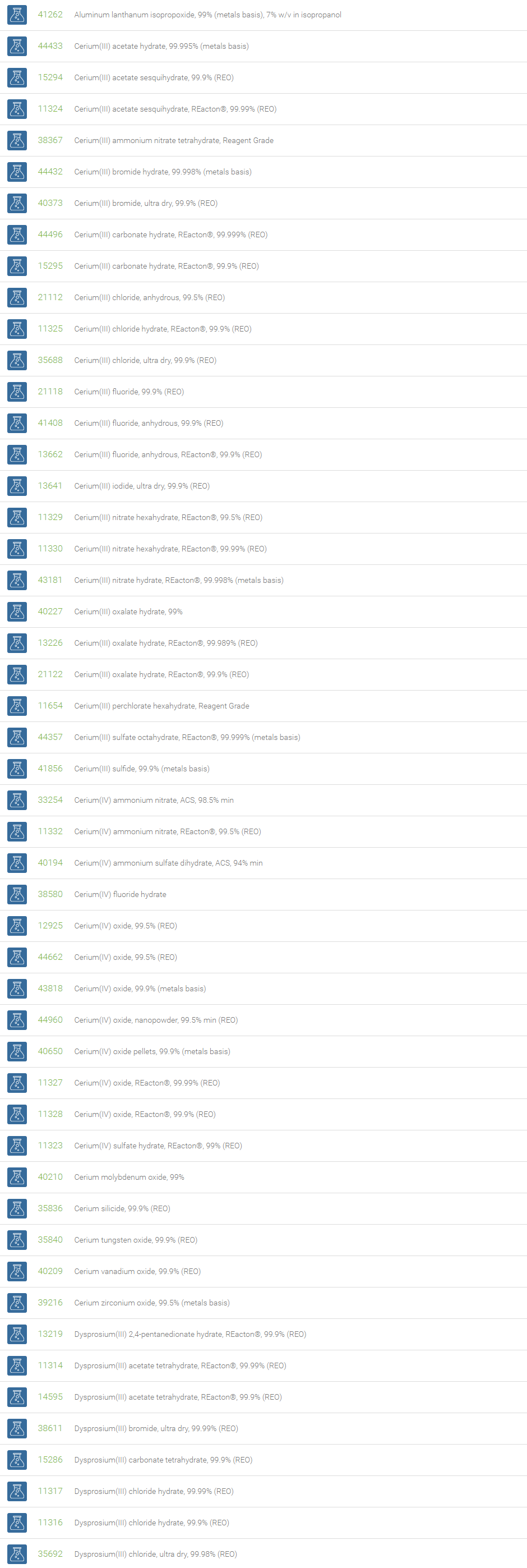
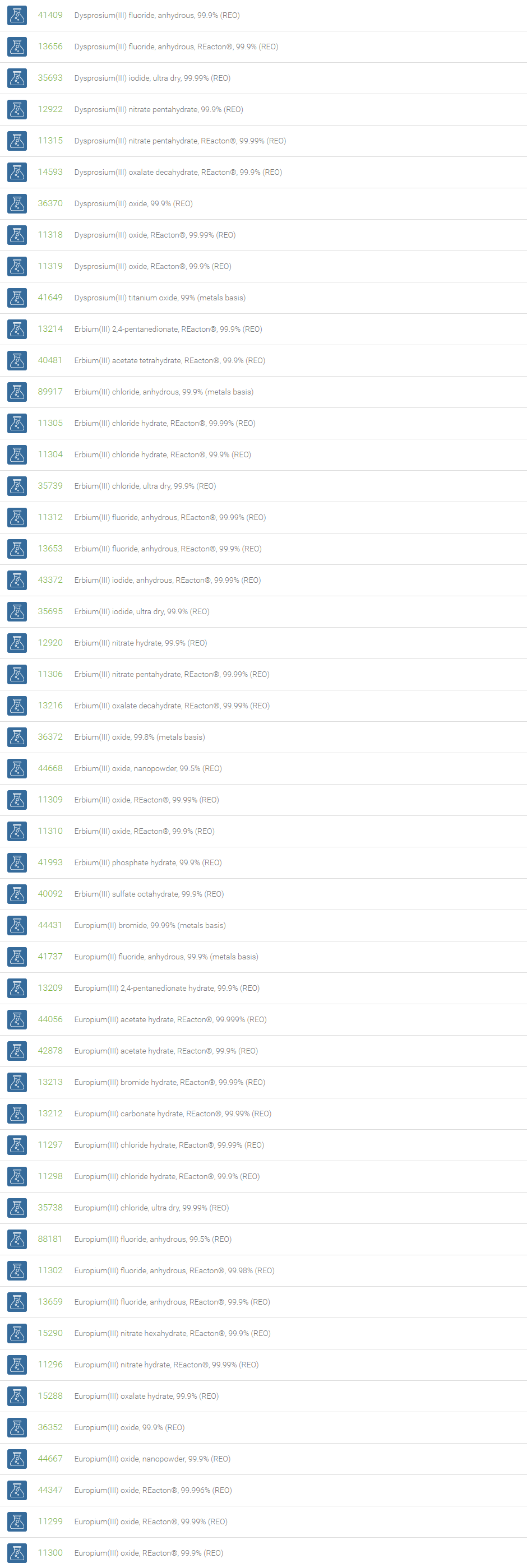
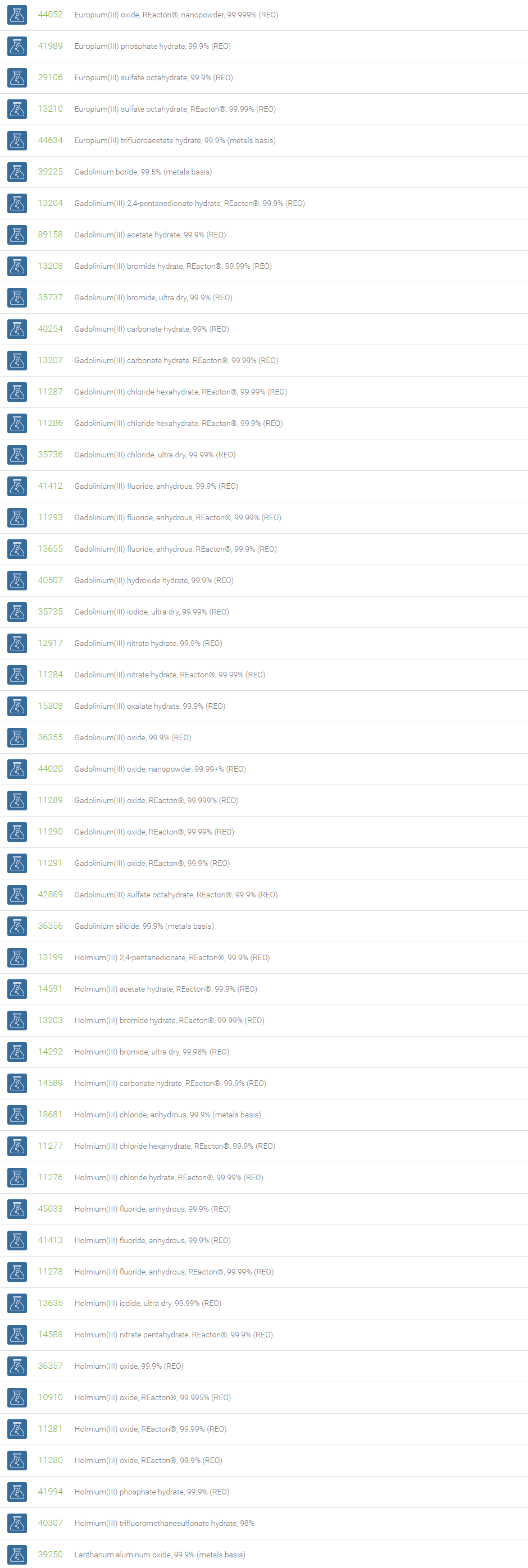
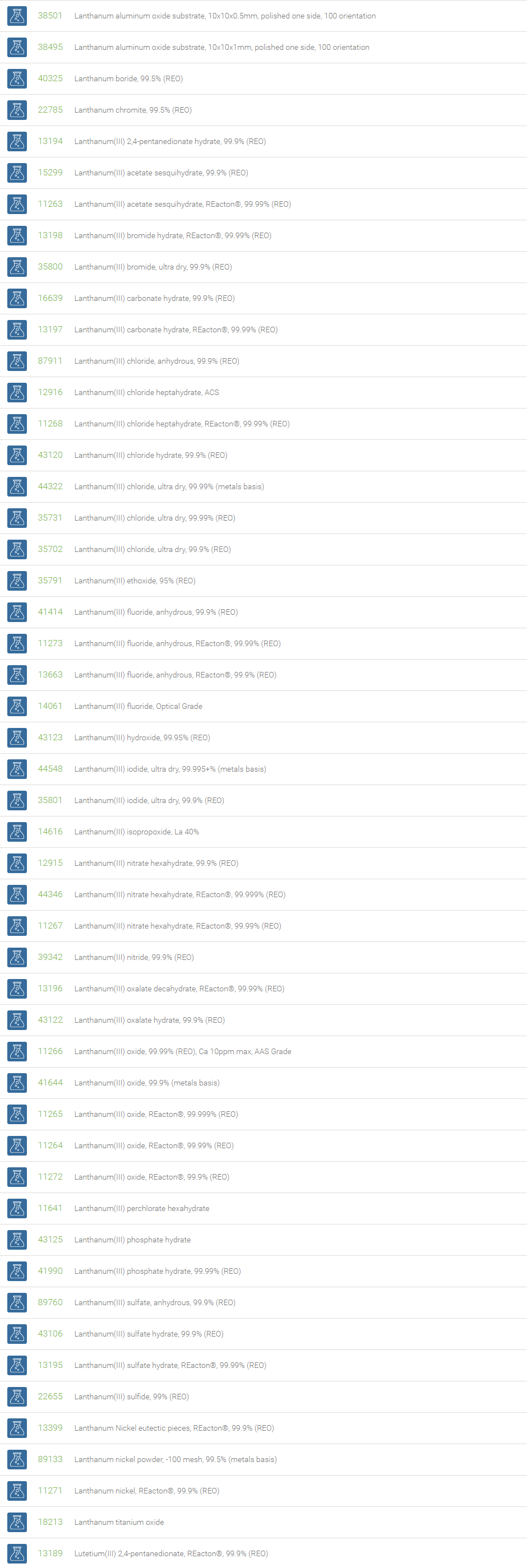
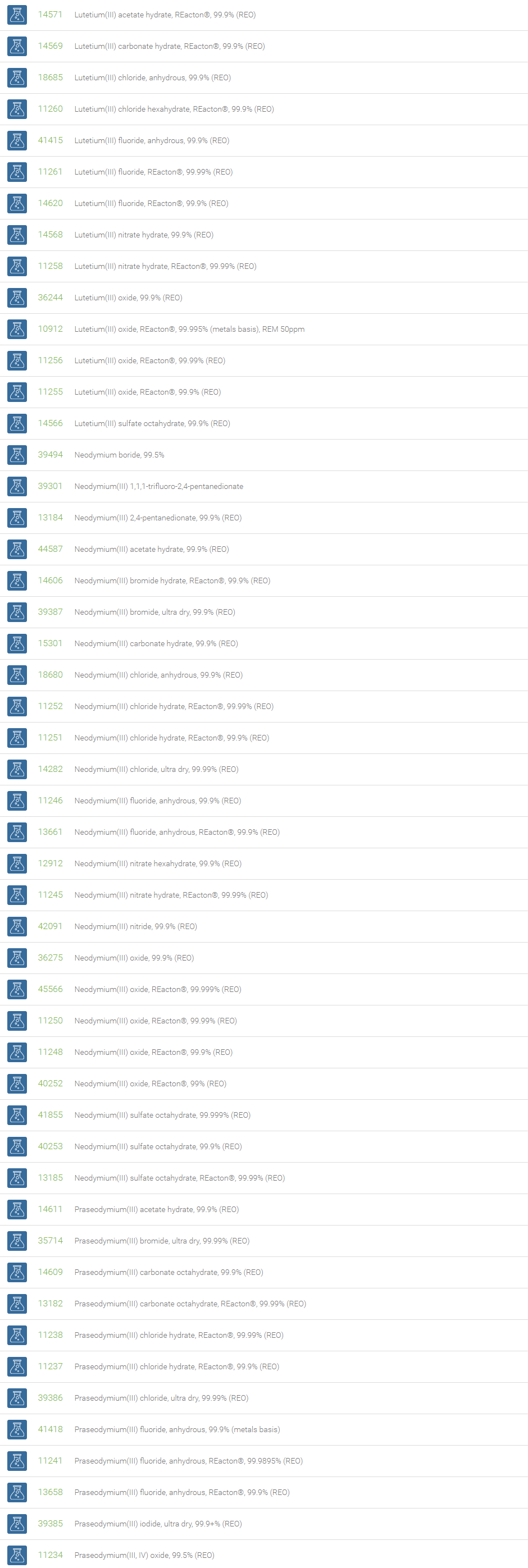
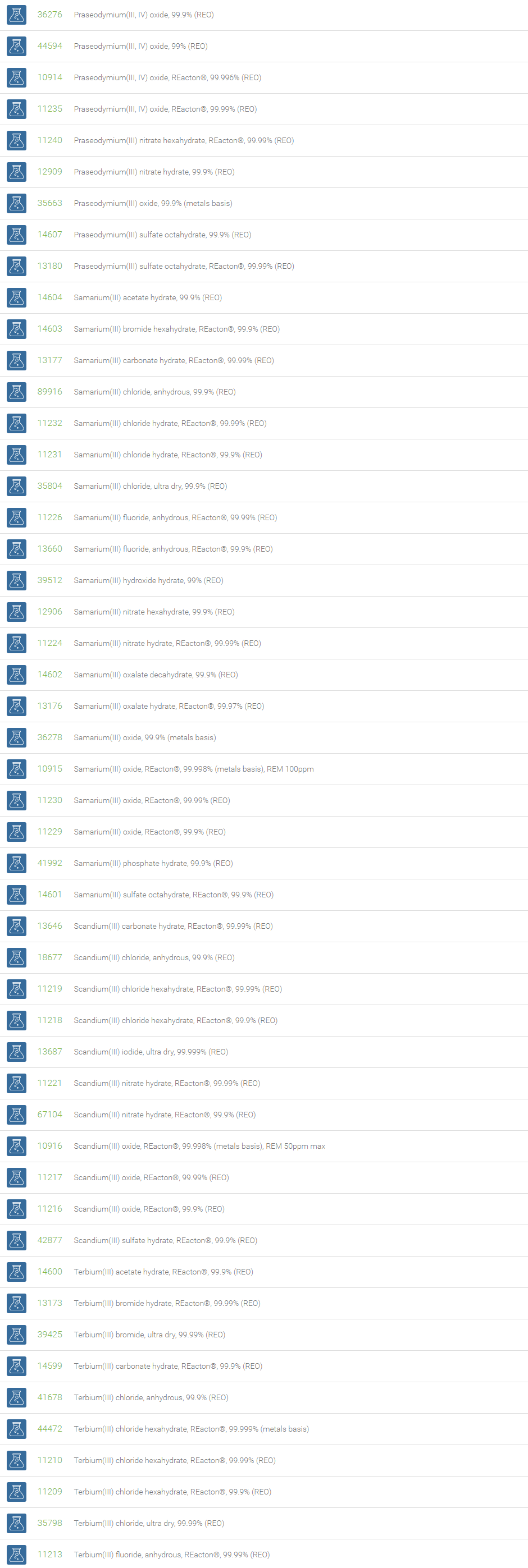
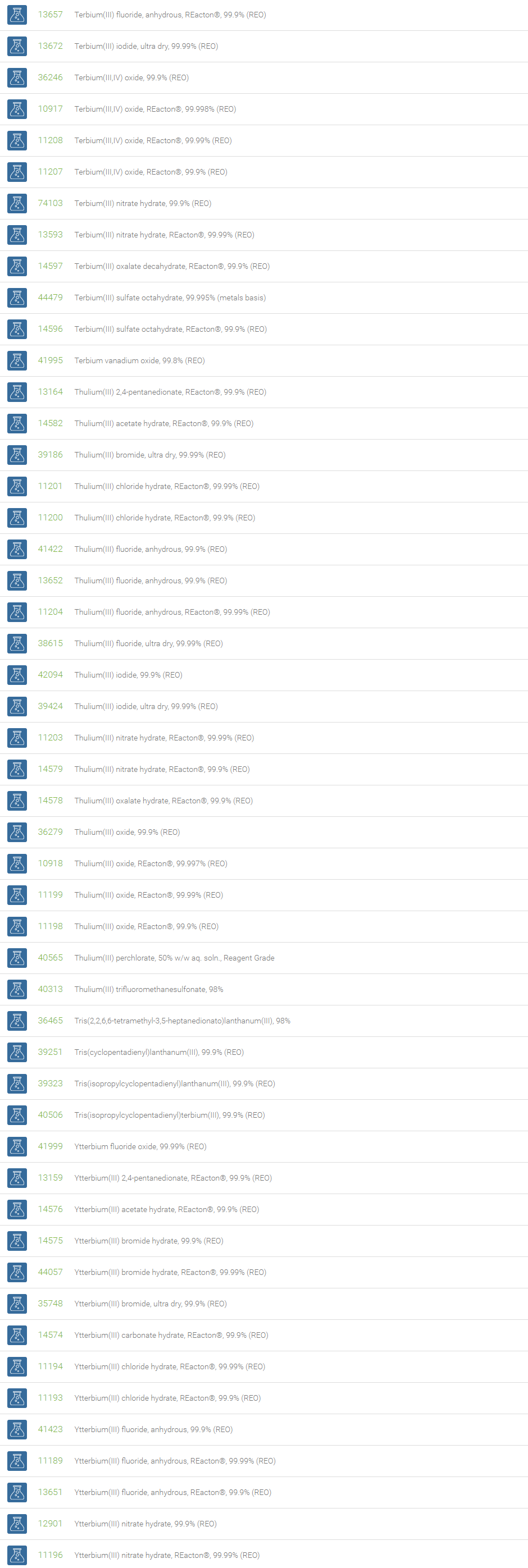
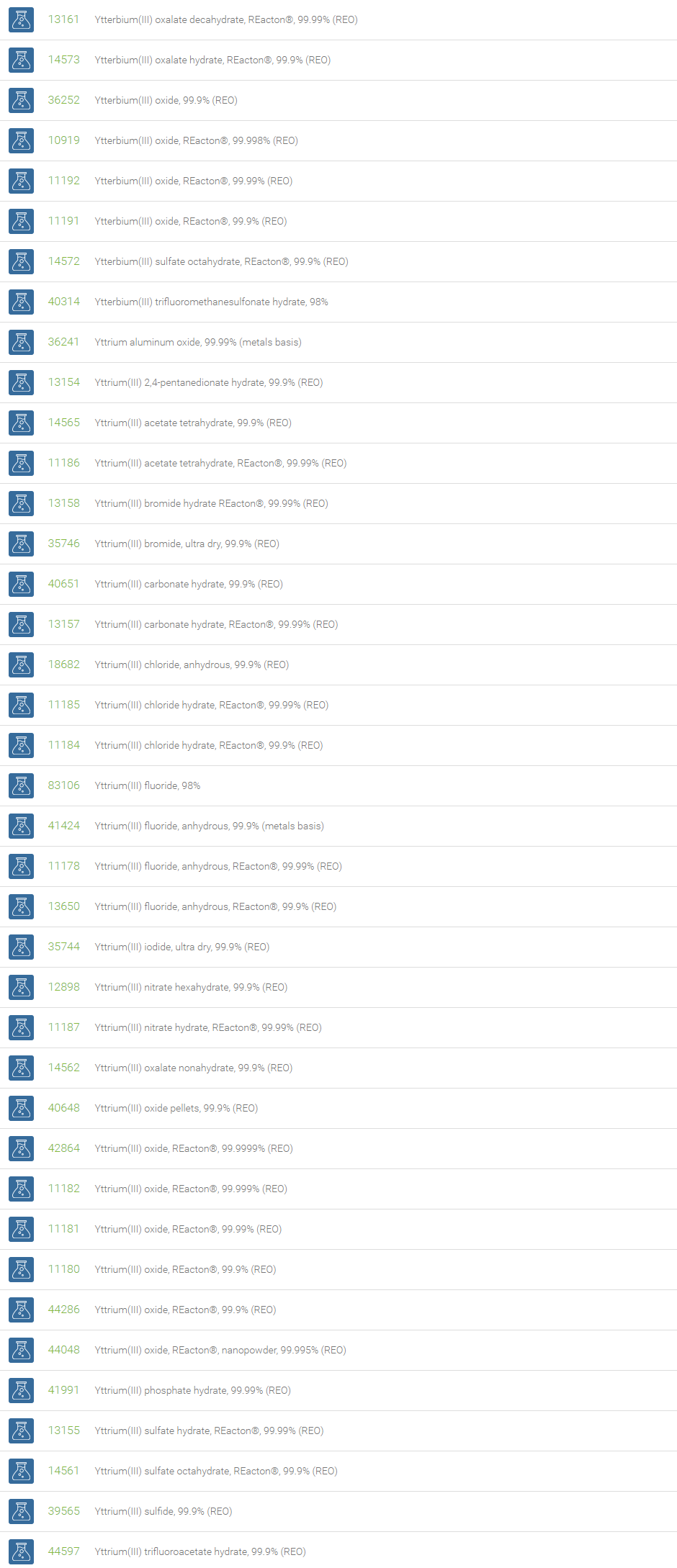
Rare Earth Compounds

Rare Earth Compounds
Rare earth elements are found in the Earth’s crust. Because of their unique magnetic, luminescent, and electrochemical properties, as well as durability, and thermal stability, these elements play a crucial role in electronics, communications, advanced aviation, health care, and military hardware.
The lanthanide series of elements and two other elements (Scandium and Yttrium) that share similar chemical properties are considered to be rare earth elements. The main uses of non-lanthanide rare earth elements include color television and fluorescent energy-saving lamps, superconductors, powerful pulsed lasers, and camera lenses. They are also useful in cancer treatment drugs, rheumatoid arthritis medicines, and surgical supplies.
Some lanthanides and their isotopes are used in pacemakers (promethium), to kill cancer cells (samarium), to target tumors in neuron therapy (gadolinium), in the treatment of certain cancers (ytterbium) and to target certain types of tumors (lutetium).
Lanthanum is used to make infrared absorbing glass, camera and telescope lenses, and also useful in petroleum refining. Cerium oxide is used as a catalyst in catalytic converters in automotive exhaust systems to reduce emissions. Praseodymium is primarily used in rare earth magnets and high-strength metals for aircraft engines. Neodymium is used to make infrared lasers for industrial and defence applications. Samarium is part of very powerful magnets used in many transportation, defense, and commercial technologies. Europium and Terbium are used to create visible light in fluorescent bulbs and in color displays in television sets. Dysprosium is often added to permanent rare earth magnets such as Holmium to help them operate more efficiently at higher temperatures. Erbium is a key component of high-performance fiber optic communications systems. The isotopes of Thulium are widely used as the radiation device in portable X-rays. Ytterbium has found applications in monitoring the effects of earthquakes and explosions on the ground. Lutetium has applications in petroleum refining and positron emission tomography. Lutetium isotopes can help reveal the age of ancient items, like meteorites.
Gadolinium is suitable for shielding in nuclear reactors and neutron radiography. Holmium can be used in nuclear control rods and microwave equipment. Samarium, dysprosium, and erbium can also be used in neutron-absorbing control rods. All of the rare earth elements contribute to vital technologies that provide safety, health and comfort to mankind.
Neodymium is used with praseodymium to create some of the strongest permanent magnets. These magnets are used in modern aircrafts and consumer electronic goods such as headphones, microphones and computer discs.